Serology testing is a term that has been floating around headlines and hospitals for several weeks as a potential big next step in our COVID-19 response. With the ability to look deeply into the population’s level of immunity, serology testing is a key tool to address important issues as we look to open up the economy, return to work, prepare for a vaccine and move past this pandemic.
Serology measures a person’s levels of antibodies, called immunoglobulins, created as an immune response to an invader. Serology testing does not detect the presence of the SARS-CoV-2 virus itself, but rather detects the antibodies that are, or were, produced as part of the body’s natural response to fight the infection.
Antibodies are proteins produced by the immune system in response to an infection, and they are specific to that particular infection. They are found in the liquid part of blood, called serum or plasma, and our body produces different types of antibodies based on the time of infection.
What do IgG and IgM tell us?
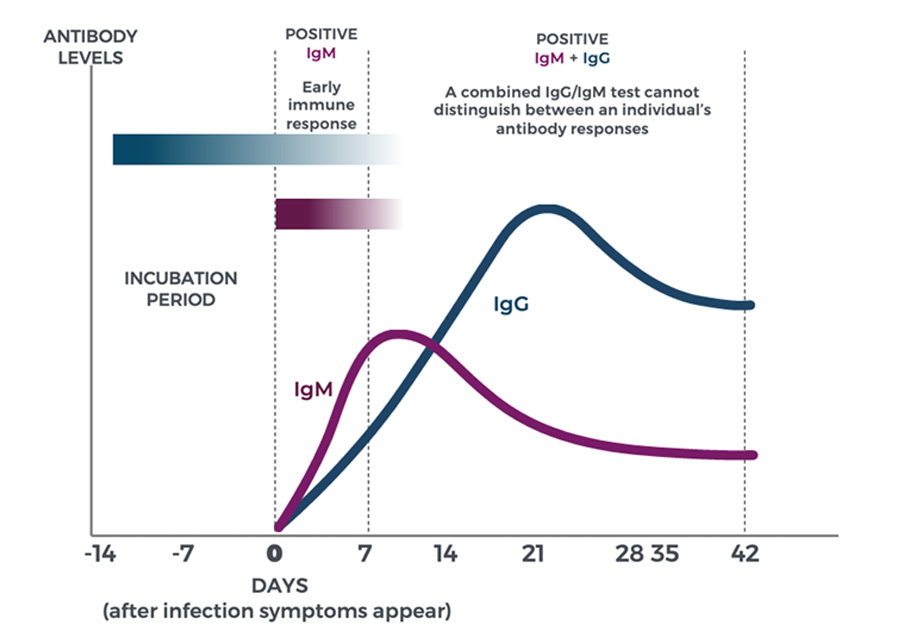
Source: Partners In Health Guide | COVID-19. Part I: Testing, Contact Tracing and Community Management of COVID-19. Updated 20 March 2020. https://www.pih.org/sites/default/files/2020-03/PIH_Guide_COVID_Part_I_Testing_Tracing_Community_Managment_3_21.pdf [Accessed: May 7, 2020]
Immunoglobin M (IgM) antibodies are produced against an antigen in the early stages of infection and are detectable after four to seven days. Immunoglobin G (IgG) antibodies are produced seven to 14 days after infection, and are detectable for months and even years, depending upon the antigen and the individual. While IgM antibodies are short-lived and may indicate that the virus is still present, IgG antibodies are more durable and could be the key to lasting immunity.
What do SARS-CoV-2 IgG and IgM tell us?
Since SARS-CoV-2 is a new virus, we are still learning how our immune response works against COVID-19 and exactly how long antibodies last.
Total IgG and IgM assays cannot distinguish between early (IgM) and late (IgG) antibody responses, and as a result, don’t provide a clear picture about whether an individual has potentially developed a longer-term immune response (IgG) or is currently infected (IgM). Alternatively, an IgG-specific serology test reveals if a person had coronavirus in the past and has developed antibodies that are highly specific to the virus.
While we don’t yet know if IgG antibodies offer lasting SARS-CoV-2 immunity, the IgG-specific test does tell clinicians of past infection, which can provide important information regarding individual and population immunity levels.
A Combined IgG-IgM test = More Re-testing
A combined IgG/IgM test cannot distinguish between early (IgM) and late (IgG) antibody response. A positive result in the combined assay may prompt a clinician to reflex – testing again with a PCR test, IgG test or both.
A Combined IgG-IgM test = Higher Total Costs
Based on example workflows
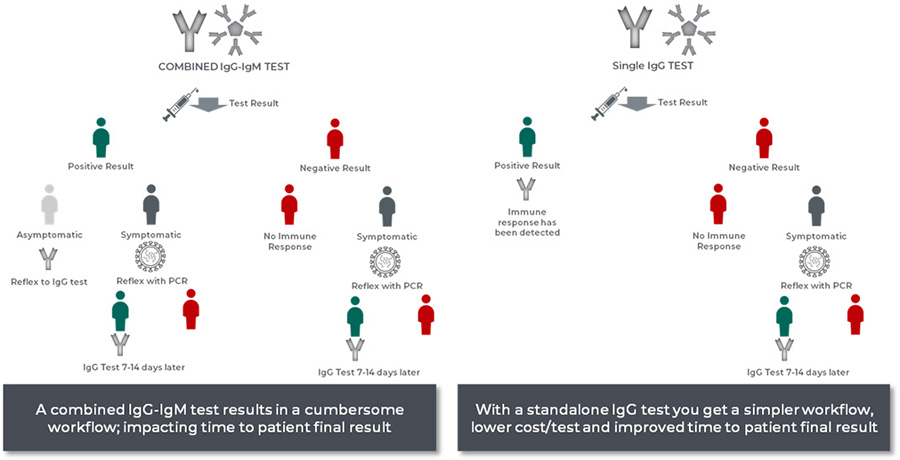
The medical community requires accurate and precise tests to make informed decisions. The right serology testing will help us find the answers we need to decrease public health risk, contain the spread, identify those who are able to work more safely on the front lines as we battle the ongoing COVID-19 pandemic and ensure treatment is properly allocated.
Discover more about the Access SARS-CoV-2 IgG assay.

 English
English