More than 620 million people are currently living with cardiovascular disease (CVD), and that number is on the rise;1,2 between 1990 and 2022, deaths from CVD increased from 12.4 million to almost 20 million.3 Each year, millions of patients present to Emergency Departments (EDs) with chest pain.4 Differentiating chest pain due to cardiac ischemia from benign conditions is challenging.5 Suspected acute myocardial infarction (AMI) is a leading cause of trips to the ED, and cardiovascular diseases remain a primary cause of death.6 For a condition where minutes matter, identifying a rapid, reliable biomarker to accurately identify AMI is necessary to reduce mortality and improve patient outcomes.7–9
The road to a high-sensitivity cardiac biomarker
In the 1970s and 1980s, CK-MB, creatine kinase-myocardial band, became the standard for identifying AMI. Creatine kinase (CK) is an enzyme present in all muscle cells, but the MB (myoglobin-binding) isoenzyme primarily occurs in cardiac muscle cells.10 When the heart is damaged, for example, during a heart attack, CK-MB leaks into the blood, where levels quickly climb. CK-MB levels peak about 12 hours after a heart attack. Prior to identification and implementation of CK-MB testing, patients with cardiac symptoms were observed for up to 3 days in the coronary care unit to determine whether they had a heart attack. With CK-MB, that time was reduced from days to hours.11
As useful and revolutionary as CK-MB measurements were in AMI, they were not terribly sensitive or specific. CK-MB does rise during a heart attack, but levels can also rise with skeletal muscle diseases, which decreases its usefulness. A new cardiac biomarker was needed.
What is cardiac troponin?
Troponin, another protein found in cardiac muscle, serves as a marker for cardiac damage. Troponin is a complex of three regulatory proteins (troponin C, troponin I, and troponin T) integral to contraction of skeletal and cardiac muscle. In the 1990s, cardiac troponin I (cTnI) was identified as a valuable biomarker for cardiac ischemia. Replacing CK-MB with troponin for MI detection has increased the ability to detect myocardial damage from heart attack up to 130%12—troponin assays, especially the newer, high sensitivity troponin assays, are not only more sensitive but also more specific than CK-MB assays.12
In a healthy person, circulating levels of cardiac troponin are extremely low, but like CK-MB, during myocardial infarction, troponin is released from the damaged myocardium.13 Troponin levels peak around 24 hours after a heart attack. With the older, low sensitivity troponin testing, a repeat test was needed 6-9 hours after presentation,5 but in 2010, high sensitivity cardiac troponin (hs-cTn) assays became available globally, shortening the detection window to 2-3 hours. In 2017, these tests were introduced in the US.14 High sensitivity troponin testing, now the test of choice (Figure 1), enables a quick decision-making pathway for management of chest pain in the ED.14,15 The accuracy and precision of hs-cTn allows for detection of cardiac damage earlier—when the patient’s troponin levels are just beginning to rise following a heart attack.15
The International Federation of Clinical Chemistry (IFCC) issued guidance on hs-cTn assays. To be classified as a high sensitivity assay, the assay must:
- have analytical imprecision ≤ 10% CV at the 99th percentile URL of a healthy population
- be able to measure cTn above the Limit of Detection (LOD) in ≥ 50% of a healthy population16
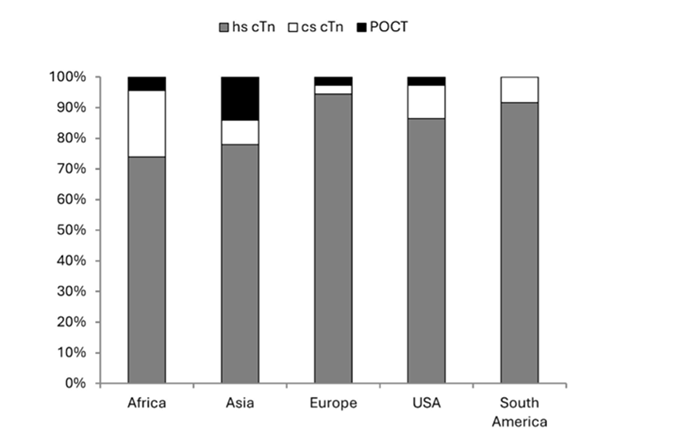
Figure 1. Troponin testing method in the main laboratory by geographic region. From Collinson et al. 202415
These rapid algorithms require high sensitivity troponin assays with excellent accuracy and precision at the lower end of detection—when the patient's troponin levels just start to rise after a cardiac event occurs. Today, hs-cTn is the biomarker of choice for chest pain management used in Emergency Departments around the world.15
Troponin and Sex†
Understanding the impact of sex† on the presentation and interpretation of hs-cTn results is essential for providing personalized and effective care. Females make up more than half of those with CVD,1,17 but they often remain “understudied, underdiagnosed, and undertreated,”17 which may lead to missed diagnoses in typical cardiac workups.17
And while overall mortality from coronary artery disease is steadily decreasing, there has been an increase in younger patients (35-54 years old), especially among females with the most significant increase in black females.18
Symptoms of heart attack in females are still often considered “atypical”19–21 when in fact they are absolutely typical—for a female. As one group put it, in the realm of females and cardiovascular disease, “Atypical symptoms were also common findings.”20 Interestingly, females are significantly more likely to present later to the hospital (≥12 hours from symptom onset) and more likely to be suspected of a non-ischemia problem than their male counterparts.22
Females show lower troponin levels, largely because they tend to have smaller hearts23–25—using a universal troponin cutoff value may result in under diagnosis in females. Sex-specific troponin ranges are only possible due to the high sensitivity and specificity of cardiac troponin assays, which allow for detection of even slight changes and enable a better understanding of myocardial injury, especially in female patients.26 When sex-specific thresholds for hs-cTn were studied, the proportion of males and females showing myocardial injury was shown to be roughly equivalent.24
Using hs-cTn testing has reduced the time to rule in or rule out cardiac ischemia for all patients,5 while expanding initiatives to educate women, especially women of color, about heart disease may help raise awareness, increase heart-health literacy, and decrease the incidence of heart disease in this highly vulnerable group.15,27
In 2022, the Clinical Laboratory Improvement Amendments (CLIA) program—recognizing the changes in precision and accuracy of clinical laboratory tests and availability of newer assays—added 29 new regulated analytes. Troponin testing is part of the class of 2024,*28,29 which requires more rigorous CLIA standards and demands greater precision and accuracy to improve safety for all patients.30
When Westgard QC independently verified the Access hs-cTnI assay on the DxI 9000 analyzer to test its accuracy and reliability, the results were outstanding.

 English
English