
ABSTRACT
Our goal was to evaluate the effectiveness of the Total Laboratory Automation system DxA 5000 with REMISOL Advance by Beckman Coulter (DxA 5000) in terms of laboratory turnaround time (TAT) and sample manual handling.
Through data collection on specific metrics within the laboratory workflow, we compared and analyzed TATs pre- and post-installation of the DxA 5000. We also compared the rate of pre-analytical errors, and the completion time of the last sample of the day in this study, setting to better understand the efficiency and productivity of the new automatic system.
Our results show that the new DxA 5000 solution can deliver a rapid and consistent turnaround time, together with a new level of comprehensive pre-analytical sample quality detection, reducing the number of manual processing steps and improving laboratory efficiency.
Article also available in German, Spanish, French and Italian.
INTRODUCTION
Diagnostic blood testing is the most commonly performed clinical procedure globally, and impacts most of the medical assessments made in the clinics and in laboratory settings.1 Since the mid 1970s, laboratory automation has been the cornerstone of many clinical laboratory services, and over time this field has witnessed major technological transformations.2 The development of flexible laboratory automation systems has attracted tremendous attention in recent years, as biotechnology scientists perform more diverse types of protocols in hospital settings; and, move to speed-up the workflow while maintaining the quality and accuracy of the results.3
What characterizes “Total Laboratory Automation” (TLA) is the ability to integrate all steps of sample processing from pre-analytics to post-analytics with sophisticated robotics and software.2 The latest consolidation of analyzers derived from multiple disciplines onto a single system is able to provide a greater scope of efficiency, with benefits that reach beyond laboratory services: improving quality and accuracy of test results; and also delivering a more effective clinical service.
It is well known that Quality Assurance (QA) and Quality Control (QC) play important roles in assuring compliance to current good manufacturing practices (cGMP), safeguarding the consistency and quality of the test results.4 But, another important aspect of clinical laboratory quality is timeliness, which is expressed as turnaround time (TAT).5 Currently, the term “therapeutic TAT” is used to describe the interval between when a test is requested to the time a treatment decision is made.6,7 Laboratory services aim to provide a rapid, reliable, and accurate report for clinicians, based on a prompt service of effective communication systems and rapid turnaround of test results, so that the time “vein-to-brain” is effective and patient treatment is not delayed. As such, TAT is now one of the most noticeable badges of laboratory service, and is often used as a Key Performance Indicator (KPI) of laboratory performance.7
In the actual laboratory setting, the definition of TAT is usually restricted to intra-laboratory activities, which separate the sample handling steps into different workflow phases:
1. Pre-analytical (order to preparation)
2. Analytical
3. Post-analytical (reporting to action).8
In each phase, there are critical points for TAT analysis that can be used to examine the efficiency of TLA systems. This, together with a number of requirements needed to compare the data collected, can comply a uniform study on the efficiency of a TLA.
As such, our objective with this study was to show through routine core lab analysis metrics, the effectiveness of the new TLA DxA 5000 solution from Beckman Coulter in terms of reduction of laboratory TAT and specimen manual handling during clinical laboratory peak times.
METHODS
Requirements for comparable data collection
In order to be able to compare the data collected appropriately, a set of standards were defined by the research team accordingly, providing templates for data recording as far as possible. The start and endpoint of the measurements within the process were outlined, together with the data source. Blood Science sample tubes chosen for analysis were STAT (emergency), Citrate, and Routine Serum. Further clarifications were made regarding whether the surveys were manual or automatic.
In order to evaluate TAT during the busiest period of the laboratory, times have all been collected in one day during peak hours, 12:30 – 16:30. As such, TAT data was collected on 2.07.2019 in the pre-installation; and, on 7.10.2020 on post-installation setting. Analysis was made with median values calculated from raw data.
TAT measurements pre-installation of the DxA 5000 (manual handling process)
Since the samples arrive in the laboratory in large batches per driver, all samples of the batch were given an identical arrival time once they reach the lab (driver entry time).
In the case of the PRE-installation study protocol, manual and random samples were identified upon laboratory arrival and tracked until the end of the analytical process. The measurement started each time from the point when a driver entered the lab with his tour. Researchers manually tracked the randomly taken samples until the input measurement of the first automation platform. Next, researchers gathered time stamps from multiple sources (Automate (standalone sample sorter), analytical platforms, data management system), that indicate each of the next steps in the process. This time stamps were then combined to evaluate the total time it takes from entry of the sample in the laboratory until the results are available for clinical use.
TAT measurements post-installation of the DxA 5000
In the post-installation study protocol, manual and random samples were identified upon laboratory arrival and manually tracked until they were introduced into the DxA 5000 system. From this point the sample was tracked through REMISOL Advance, the data management software included with DxA 5000, to the end of the analytical process. As such, as described above, all samples arriving in the same batch were given an identical arrival time (driver entry time) and the final time upon completion of the analysis was recorded and used for the evaluation of TAT.
Error detection
The error detection data was collected on a single day in pre- and post-installation settings. Wrong tube types, insufficient sample volume, serum indices via connected analyzers, or barcode errors were tracked manually in the pre-installation setting; and, automatically through DxA 5000 with REMISOL Advance software, in the post-installation setting.
Last delivery data analysis
In order to evaluate worker and systems productivity in the pre- and post-installation settings, the time was recorded for the last delivery of the day, and then the time taken to complete the last sample tube of the day in both settings. The data was collected from March-May 2019 in the pre-installation; and, from July-September 2020 post-installation setting.
RESULTS
DxA 5000 reduces total turnaround time (TAT) in multiple sample types receiving by a typical blood sciences laboratory
TAT was analyzed and compared pre- and post-installation of the new TLA, DxA 5000. As described in the methods section, TAT was calculated manually or automatically since samples arrived in the laboratory until the result was ready for clinical use (total TAT). As seen in Figure 1, during laboratory peak times there is a significant reduction in total TAT in handling different types of core samples (STAT, Routine Serum and Citrate). As such, after the installation with DxA 5000 there was a 61% reduction in total TAT for emergency samples (STAT), 12% reduction in total TAT for routine serum tests, and 53% reduction in total TAT for citrate tubes.
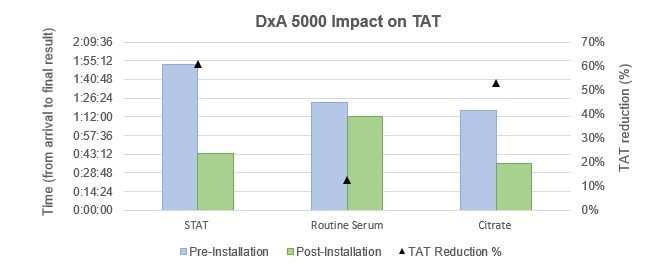
Figure 1 – Left axis: Comparison of the time taken between arrival and final result availability in different laboratory samples (STA, Routine Serum and Citrate) in the settings of pre- and post-installation of DxA 5000. Right axis: Analysis of the impact on total turnaround time (TAT) percentage reduction pre- and post-installation of DxA 5000.
We defined laboratory-controlled TAT for the most time-sensitive specimens from the time of accessioning in the laboratory to the release of the results into the electronic medical record at laboratory peak times. Using this criterion, our data indicated that there is an effective reduction in sample handling time post-installation with DxA 5000. Since TAT is a measure of functionality and effectiveness of a laboratory service, what these results show is that with the automated DxA 5000, the laboratory workflow becomes more efficient providing clinical results faster. Because clinical decisions depend on quick laboratory result reporting, the timeliness of the automated DxA 5000 TLA speeds clinical decisions, which in turn affects patient outcome and, consequently, saves lives.
DxA 5000 enhances quality and efficacy of laboratory diagnostic testing
As can be seen in Figure 2, after the samples are accepted in the laboratory, the automation of the identification of pre-analytical error resulted in an increase sorting of defective samples. Whether through identification of wrong tube types, insufficient sample volume or barcode errors through DxA 5000, or serum indices via connected analyzers, the automated DxA 5000 system was able to identify more defective samples in comparison with a manual handling system. Setting decision rules based on predefined criteria with REMISOL Advance software allowed the auto verification of data, automatic re-analysis of samples with highly abnormal or suspect results, as well as triggering reflex (reflective) and add-on testing, thus ultimately contributing to enhanced quality and safety of diagnostic testing.

Figure 2 – Comparison of the identification of Pre-Analytical Error in different types of samples pre-installation (left – 4% of tubes identified with an error; data collected on a single day) and post-installation (right – 8% of tubes identified with an error ; data collected on a single day) with DxA 5000.
Also, with the automated DxA 5000, samples were automatically archived and available for further analysis at the end of the run, conducting to a decrease in manual handling of the samples, which further enhances the efficiency of the laboratory workflow. Similarly, through patented innovations in intelligent sample routing, the DxA 5000 solution allowed for better sample management, enabling auto-verification of data, automatic re-analysis of samples with highly abnormal or suspected results, without the need to wait to re-run all samples in one batch. Furthermore, specimen traceability is consistently enhanced by maintaining all routine and STAT samples within a unique environment, enabling digital traceability of all the processes a tube has been subjected to, from time of delivery to the laboratory, up to storage once testing has been completed.9 This conclusively contributes to enhanced quality and efficacy of laboratory diagnostic testing.
The last tube of the day is analyzed quicker with DxA 5000
As can be seen in Figure 3, during the three-month period of analysis, the manual handling of the last sample of the day would take around 1h:20min to complete (pre-installation). Post-Installation with DxA 5000, the last tube of the day was always analyzed in less than 60 min, conducting to an effective decrease in the time it takes to deliver a clinical result at the end of the working day. There is a 20% decrease in time in the first month of analysis, 61% reduction on the following month, and 38% less time is needed to complete the analysis of the last tube of the day post-installation with DxA 5000.
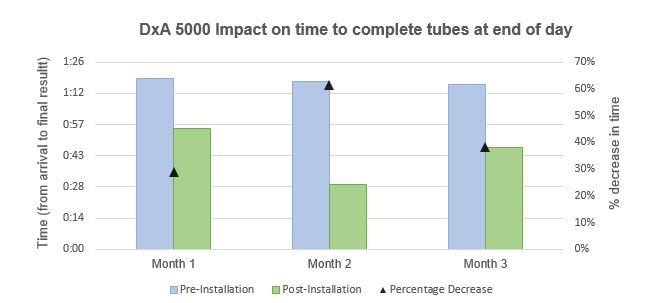
Figure 3 – Left axis: Comparison of the time taken between arrival and final result availability of the last tube of the day in the settings of pre- and post-installation with DxA 5000. Right axis: Analysis of the percentage decrease in time taken to complete the analysis of the last tube of the day post-installation with DxA 5000.
With the new DxA 5000, the last batch of the day is done quicker than with manual handling. This fact leads to a less burdened workforce, ultimately increasing morale and enhancing the productivity of the staff. In today’s view of mental health awareness, effective ways of promoting an improved work-life balance will lead to a further commitment and enthusiasm from the worker-side.10
DISCUSSION
In the frontier of clinical diagnostics technology, it is clear that the integration and automation of systems is key to enable a rapid, reliable and accurate laboratory service. The prototypical TLA is the conjunction of many analyzers performing different types of tests on diverse sample matrices, physically integrated as modular systems or connected by assembly lines. The opportunity to integrate multiple diagnostic specialties into one single track aims to improve efficiency, organization, standardization, quality and safety of laboratory testing, whilst also providing a significant long-term return on investment and enabling staff reduction or requalification9. As such, TLA systems have expanded globally due to the growing recognition of their value in improving the efficiency of laboratory services. The new DxA 5000 cuts-back the total laboratory TAT needed for sample analysis and further increases quality control. With this new automated system, manpower can now be redirected towards value-added activities such as quality assurance - allowing for a redefinition and requalification of the job roles with increased worker skills. Not only that, but the DxA 5000 reduced the manual handling requirement needed within the laboratory, preventing technicians moving back and forth from one analyser to another, therefore minimizing the distance covered by the personnel for performing multiple analyses on different instrumentation and diminishing the risks of handling biohazardous material. On top of that, it reduces the time analysis taken for the last delivery of the day, performing this task quicker than with manual handling. This ultimately leads to a less burdened workforce, enhancing the staff work-life balance and supporting productivity. Our study, of course, has certain constraints, since it would be interesting to evaluate where specifically in the laboratory workflow the time gained is grasped. For example, in the pre-analytical phase that includes intense sample handling, many important errors can occur due to manual mislabelling or other oversights. Since these errors occur upstream of the workflow process, many times these errors follow down the laboratory roadmap undermining the process and demanding re-runs that could be avoided otherwise. As such, the pre-analytical phase must have rigorous control measures in the reception/registration units to avoid inadvertently allowing problems or errors to travel further "downstream". With this in mind, we hope to conduct a new study to evaluate TAT per laboratory phase (pre-analytical, analytical, and post-analytical) using the same pre- and post-installation background. This new study could reveal further improvements in the pre-analytical phase, for example, that we were not able to measure at this point. Also, it would be important to evaluate statistically the differences seen, but since we had many more automated sample results then manual ones, we considered it would be misleading to compare different group sizes. As such, we hope to publish soon a new updated study with more specific results in terms of the differences seen between manual handling versus automated DxA 5000 with REMISOL Advance settings.
CONCLUSION
There is a new way to manage increasing workload demands, reduce errors, enhance laboratory performance and improve clinical service with the new automated DxA 5000 with REMISOL Advance. The data collected in our study indicates that a new standard in total turnaround time and sample quality control can be achieved in the clinical laboratory with this new TLA system.
 English
English

