1. Rudd KE, Johnson SC, Agesa KM, et al. Global, regional, and national sepsis incidence and mortality, 1990-2017: analysis for the Global Burden of Disease Study. Lancet. 2020;395(10219):200-211. doi:10.1016/S0140-6736(19)32989-7
2. Martínez ML, Plata-Menchaca EP, Ruiz-Rodríguez JC, Ferrer R. An approach to antibiotic treatment in patients with sepsis. J Thorac Dis. 2020;12(3):1007-1021. doi:10.21037/jtd.2020.01.47
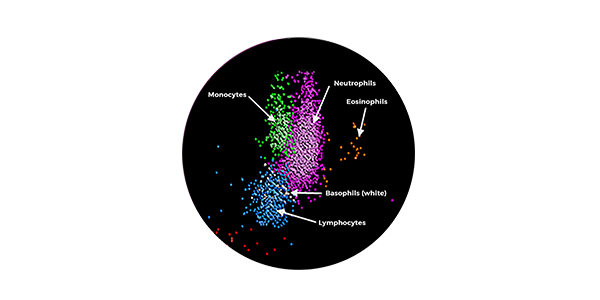
3. Fact Sheets | Sepsis Alliance. 2023. Accessed August 10, 2023. https://www.sepsis.org/education/resources/fact-sheets/
4. Perman SM, Goyal M, Gaieski DF. Initial emergency department diagnosis and management of adult patients with severe sepsis and septic shock. Scand J Trauma Resusc Emerg Med. 2012;20:41. doi:10.1186/1757-7241-20-41
 English
English