What is phi ?
phi is three things: a simple blood test to improve early detection of prostate cancer, a tool to reduce negative biopsies and provide more confidence in your biopsy decisions, and it is used as a recommendation by the National Comprehensive Cancer Network (NCCN) Guidelines for PCa Early Detection prior to transrectal ultrasound (TRUS) guided biopsies.
phi comprises three tests in combination:
- PSA
- free PSA
- p2PSA
The new p2PSA assay specifically measures [-2]proPSA. The [-2]proPSA biomarker is an isoform of free PSA that was identified as the most prostate cancer-specific form found in tumor extracts.1 The PSA, free PSA and p2PSA results are combined in the Access instrument to calculate a probability of prostate cancer.
Benefits of phi Testing
The PSA test is a widely used screening tool for prostate cancer. However, given the PSA test’s limited specificity for cancer, a more precise tool is needed for prostate cancer detection. The phi score provides better risk stratification to identify patients who need a biopsy.† The appropriate use of phi can significantly modify physicians behavior patterns and improve their ability to diagnose and manage their patients. phi is another tool to help their patients decide if a biopsy is right for them.2
The power of phi
A recent study published in Prostate Cancer and Prostatic Diseases demonstrated that physicians elected to perform fewer prostate biopsies for men who presented in the diagnostic gray zone when phi testing was included in their overall routine clinical assessment. Physicians reported that phi testing significantly impacted their patient management decision in over 73% of their cases.

Only 36% of men received biopsies when phi was included in the assessment, compared with the 60% who had to undergo such procedures before phi was available.
How is the phi analysis used in clinical practice?
Because phi analysis improves specificity for prostate cancer, it fills the diagnostic gap between PSA screening and a prostate biopsy (Figure 1).
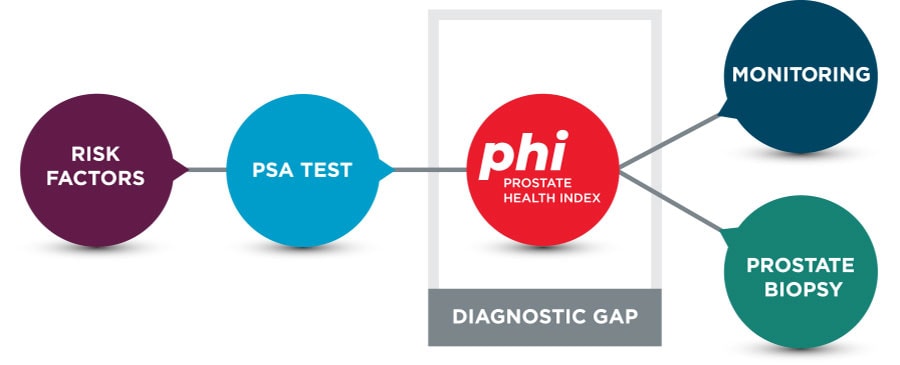
Figure 1. Diagnostic Considerations for Prostate Cancer
What does phi score mean?
Table 1 represents clinical study data analyzed to estimate an individual patient's probability of having detectable prostate cancer based on Beckman Coulter phi results when that patient has a PSA in the diagnostic gray zone between 4 and 10 ng/mL.3 At phi cutoffs between 27 to 55, the probability of cancer ranged from 16.8 to 50.1%.‡ For example, a patient with a phi result below 27 has a 90% chance that his prostate biopsy will be negative.
Table 1. Probability of Prostate Cancer Based on phi Results Between 4 and 10 ng/mL1
| phi Range* | Probability of Cancer | 95% Confidence Interval |
| 0-26.9 | 9.8% | 5.2%-15.4% |
| 27.0-35.9 | 16.8% | 11.3%-22.2% |
| 36.0-54.9 | 33.3% | 26.8%-39.9% |
| 55.0+ | 50.1% | 39.8%-61.0% |
Clinical study data shows phi results can be used to assess probability of cancer.
The phi diagnostic gray zone in countries outside of the U.S.and China is defined as PSA levels between 2 and 10ng/mL. The probability slightly changes based on these PSA levels (see Table 2).
Table 2. Probability of Prostate Cancer Based on phi Results Between 2 and 10 ng/mL2
| (Hybritech Calibration of PSA and free PSA) | ||
| Beckman Coulter phi Range* (Hybritech Calibration) |
Probability of Cancer | 95% Confidence Interval |
| 0-21 | 8.4% | 1.9%-16.1% |
| 21-40 | 21.0% | 17.3%-24.6% |
| 40+ | 44.0% | 36.0%-52.9% |
Clinical study data shows phi results can be used to assess probability of cancer.
 English
English






