Diagnostic Uncertainty
Clinicians are faced with time pressures to make early risk stratification decisions for patient disposition and treatment initiation. Clinicians must triage patients and quickly determine where they need to go. In many cases, the patients may be discharged home or, perhaps, missed to taken ICU, but rather to the general ward – and both scenarios are associated with increased mortality - higher mortality in patients discharged to the general ward (42.7%) or home (61.6%) vs. ICU (29.5%).2-3 Initial disposition home of emergency department patients with potential sepsis results in twice the mortality and 4x healthcare costs per patient.1
Diagnostic uncertainty is a major challenge for patients presenting to the emergency department with a low number of signs and symptoms. Over 30% of septic shock patients that present with vague symptoms may be missed by the EMR alerts, encounter longer time to antibiotics, and experience worse outcomes.4
Mitigate Diagnostic Uncertainty
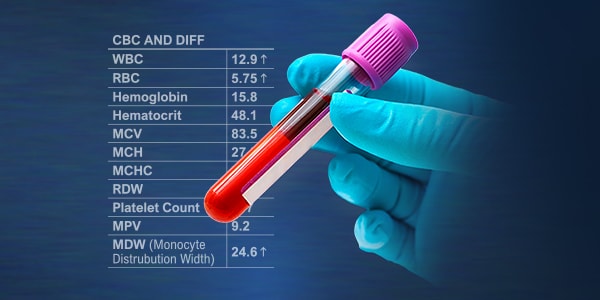
MDW is available early in-patient assessment to help determine patient acuity and risk of sepsis.

What Makes MDW Unique?
- Monocyte Activation is a common physiological signal in severe infection and sepsis
- MDW is backed by rigorously designed patient trials and several rounds of in-depth review to meet the standards required for regulatory-clearance and CE mark
- MDW helps reduce diagnostic uncertainty. It is available early to enhance the initial risk assessment and potentially shift your clinical decision point helping clinicians escalate or de-escalate care
 English
English