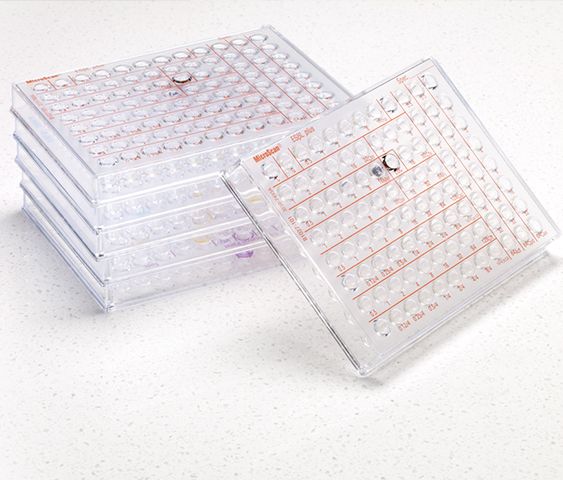
MicroScan Conventional Panels
MDROs are the cause of:
2.8 Million Infections and 35,000 deaths annually1
As the foundation of MicroScan ID/AST products, conventional panels deliver gold-standard1 accuracy through real-time detection of growth and emerging resistance. This reduces the need for expensive repeat and ancillary testing, providing timely results your laboratory can trust.
To determine which MicroScan panels best fit your needs, use our easy-to-navigate MicroScan Panel Guide. Simply choose the antibiotics that define your formulary, and our tool will produce the options that best fit your criteria.
Advance Resistance Detection and Stewardship with Direct MIC Testing
The Microscan portfolio of ID/AST panels supports unique institutional and geographic needs with a broad menu of antibiotic configurations and choice inoculum preparation methods. Our direct MIC testing methodology provides confidence for detecting subtle changes in susceptibility results. Choose the most appropriate MicroScan panels and workflow for your laboratory.
- Support a variety of formulary needs with current antimicrobials, dilutions and breakpoints
- Test both ID and AST in a single combo panel to optimize workflow
- Select MIC-only panel formats to complement MALDI-TOF ID* testing protocols
- Use ID-only panels for targeted testing needs
- Utilize PROMPT† or turbidity inoculum method for workflow flexibility
- Rely on visual confirmation of biochemical and susceptibility results for added confidence in unusual results
Product Details |
|
Download the Comprehensive panel brochure – NEW!
Download the Negative Urine Combo Panel Flyer – NEW!
Download the LabPro v5.0 flyer – NEW!
Multicenter Evaluations of Antibiotic MIC Results Using MicroScan Panels
Managing multidrug-resistant species and changing FDA antibiotic testing requirements? You can put your trust in the proven performance of MicroScan panels. A series of multicenter studies showed good correlation of MicroScan and CLSI reference panels. Find out more by reviewing the results of the studies.
Explore now
Discover a Full Portfolio of Highly Accurate Microbiology Solutions
Antibiotic resistance calls for robust and accurate microbiology solutions. Equip your laboratory with the precision and flexibility you need to detect and combat this emerging threat. Find out how MicroScan microbiology solutions can help.
The Pathway to Prescription: MicroScan Gram-negative and Gram-positive Panels
Learn more about MicroScan’s enhanced testing menu for accurate bacterial identification and susceptibility testing.
Accuracy Matters: Delivering Better Patient Care
See how to produce automated ID/AST results without sacrificing accuracy.
Biotype Lookup Program
The Biotype Lookup Program allows you to generate biotype information supported by LabPro Information Manager and Data Management System. It accesses an expanded identification database designed to assist with the identification of organisms whose atypical biochemical tests cause their assigned frequency cutoff points to be exceeded. The program supports all current MicroScan identification databases. To begin your search, select a panel classification, identification level and identification database, then enter the biotype number to obtain the requested information.
LabPro V4.42 or greater Biotype Lookup tool
PLEASE NOTE: The LabPro V4.41 Biotype Lookup tool is no longer available for use. All customers should use the V4.42 tool.
1Antibiotic resistance threats in the US 2019- CDC
*MALDI Biotyper is the property of Bruker Daltonik GmbH.†Prompt is a registered trademark of 3M.
‡Available under special contract; contact your Beckman Coulter sales representative for additional information.
§MEV vs. Best Available Therapy (BAT): 64% vs. 33%: meropenem, imipenem, ertapenem, tigecycline, colistin, polymyxin B, amikacin, gentamicin, tobramycin and ceftazidime/avibactam. Lee, Y. “Meropenem -Vaborbactam (Vabomere™): Another Option for Carbapenem-Resistant Enterobacteriaceae.” P&T® Journal. 2019 Mar; 44(3): 110 -113. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6385729. Accessed March 8, 2019.
 English
English